Tests show new biosensor can guide environmental clean ups
Device can also provide an early warning system for spills
Tests of a new antibody-based “biosensor” developed by researchers at the Virginia Institute of Marine Science show that it can detect marine pollutants like oil much faster and more cheaply than current technologies. The device is small and sturdy enough to be used from a boat.
Testing of the biosensor in the Elizabeth River and Yorktown Creek, which both drain into lower Chesapeake Bay, shows that the instrument can process samples in less than 10 minutes, detect pollutants at levels as low as just a few parts per billion, and do so at a cost of just pennies per sample. Current technology requires hours of lab work, with a per-sample cost of up to $1,000.
“Our biosensor combines the power of the immune system with the sensitivity of cutting-edge electronics,” says Dr. Mike Unger of VIMS. “It holds great promise for real-time detection and monitoring of oil spills and other releases of contaminants into the marine environment.”
The biosensor was developed and tested by Unger, fellow VIMS professor Steve Kaattari, and their doctoral student Candace Spier, with assistance from marine scientist George Vadas. The team’s report of field tests with the sensor appears in this month’s issue of Environmental Toxicology and Chemistry.
The instrument was developed in conjunction with Sapidyne Instruments, Inc., with funding from the state of Virginia, the Office of Naval Research, and the Cooperative Institute for Coastal and Estuarine Environmental Technology, a partnership between NOAA and the University of New Hampshire.
 The tests in the Elizabeth River took place during clean up
of a site contaminated by polycyclic aromatic hydrocarbons (PAHs), byproducts
of decades of industrial use of creosote to treat marine pilings. The U.S.
Environmental Protection Agency considers PAHs highly toxic and lists 17 as
suspected carcinogens.
The tests in the Elizabeth River took place during clean up
of a site contaminated by polycyclic aromatic hydrocarbons (PAHs), byproducts
of decades of industrial use of creosote to treat marine pilings. The U.S.
Environmental Protection Agency considers PAHs highly toxic and lists 17 as
suspected carcinogens.
The biosensor allowed the researchers to quantify PAH concentrations while the Elizabeth River remediation was taking place, gaining on-site knowledge about water quality surrounding the remediation site. Spier says the test was “the first use of an antibody-based biosensor to guide sampling efforts through near real-time evaluation of environmental contamination.”
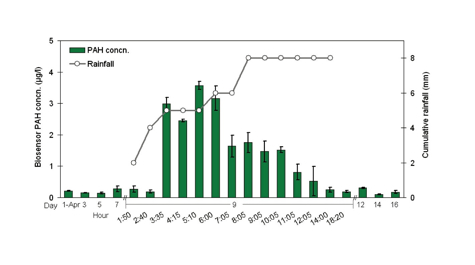
In the Yorktown Creek study, the researchers used the biosensor to track the runoff of PAHs from roadways and soils during a rainstorm.
Biosensor development
Kaattari says “Our basic idea was to fuse two different kinds of technologies— monoclonal antibodies and electronic sensors—in order to detect contaminants.”
Antibodies are proteins produced by the immune system of humans and other mammals. They are particularly well suited for detecting contaminants because they have, as Kaattari puts it, an “almost an infinite power to recognize the 3-dimensional shape of any molecule.”
Mammals produce antibodies that recognize and bind with large organic molecules such as proteins or with viruses. The VIMS team took this process one step further, linking proteins to PAHs and other contaminants, then exposing mice to these paired compounds in a manner very similar to a regular vaccination.
“Just like you get vaccinated against the flu, we in essence are vaccinating our mice against contaminants,” says Kaattari. “The mouse’s lymphatic system then produces antibodies to PAHs, TNT, tributyl tin [TBT, the active ingredient in anti-fouling paints for boats], or other compounds.”
Once a mouse has produced an antibody to a particular contaminant, the VIMS team applies standard clinical techniques to produce “monoclonal antibodies” in sufficiently large quantities for use in a biosensor.
“This technology allows you to immortalize a lymphocyte that produces only a very specific antibody,” says Kaattari. “You grow the lymphocytes in culture and can produce large quantities of antibodies within a couple of weeks. You can preserve the antibody-producing lymphocyte forever, which means you don’t have to go to a new animal every time you need to produce new antibodies.”
From antibody to electrical signal
The team’s next step was to develop a sensor that can recognize when an antibody binds with a contaminant and translate that recognition into an electrical signal. The Sapidyne® sensor used by the VIMS team works via what Kaattari calls a “fluorescence-inhibitory, spectroscopic kind of assay.”
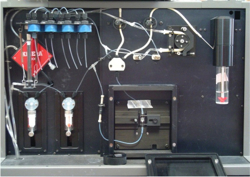
In the sensor used on the Elizabeth River and Yorktown Creek, antibodies designed to recognize a specific class of PAHs were joined with a dye that glows when exposed to fluorescent light. The intensity of that light is in turn recorded as a voltage. The sensor also houses tiny plastic beads that are coated with what Spier calls a “PAH surrogate”—a PAH derivative that retains the shape that the antibody recognizes as a PAH molecule.
When water samples with low PAH levels are added to the sensor chamber (which is already flooded with a solution of anti-PAH antibodies), the antibodies have little to bind with and are thus free to attach to the surrogate-coated beads, providing a strong fluorescent glow and electric signal. In water samples with high PAH concentrations, on the other hand, a large fraction of the antibodies bind with the environmental contaminants. That leaves fewer to attach to the surrogate-coated beads, which consequently provides a fainter glow and a weaker electric signal.
During the Elizabeth River study, the biosensor measured PAH concentrations that ranged from 0.3 to 3.2 parts per billion, with higher PAH levels closer to the dredge site. In Yorktown Creek, the biosensor showed that PAH levels in runoff peaked 1 to 2 hours after the rain started, with peak concentration of 4.4 parts per billion.
Comparison of the biosensor’s field readings with later readings from a mass spectrometer at VIMS showed that the biosensor is just as accurate as the more expensive, slower, and laboratory-bound machine.
A valuable field tool
Spier says “Using the biosensor allowed us to quickly survey an area of almost 900 acres around the Elizabeth River dredge, and to provide information about the size and intensity of the contaminant plume to engineers monitoring the dredging from shore. If our results had shown elevated concentrations, they could have halted dredging and put remedial actions in place.”
Unger adds “measuring data in real-time also allowed us to guide the collection of large-volume water samples right from the boat. We used these samples for later analysis of specific PAH compounds in the lab. This saved time, effort, and money by keeping us from having to analyze samples that might contain PAHs at levels below our detection limit.”
“Biosensors have their constraints and optimal operating conditions,” says Kaattari, “but their promise far outweighs any limitations. The primary advantages of our biosensor are its sensitivity, speed, and portability. These instruments are sure to have a myriad of uses in future environmental monitoring and management.”
One promising use of the biosensor is for early detection and tracking of oil spills. “If biosensors were placed near an oil facility and there was a spill, we would know immediately,” says Kaattari. “And because we could see concentrations increasing or decreasing in a certain pattern, we could also monitor the dispersal over real time.”